# The Ultimate Guide to Cold Chain and Logistics: 5 Critical Components for Success
Cold chain and logistics is the invisible backbone of our modern world. It is a temperature-controlled supply chain that ensures the safety, efficacy, and quality of products from the point of origin to the final consumer. Without it, we would not have fresh produce in our supermarkets, life-saving vaccines in our clinics, or gourmet meals delivered to our doors. This specialized field combines precision engineering, rigorous process management, and advanced technology. In this comprehensive guide, we will break down the five critical components that define successful cold chain and logistics operations.
Understanding the search intent behind this term is crucial. For most users, “cold chain and logistics” represents an informational query. People are looking to understand what it is, how it works, and what best practices are. They may be business owners, logistics managers, or students entering the field. This guide is designed to answer those questions in depth. We will also explore related concepts like temperature-controlled transportation, perishable goods management, supply chain visibility, and regulatory compliance.
## The Foundational Pillar: Temperature-Controlled Storage
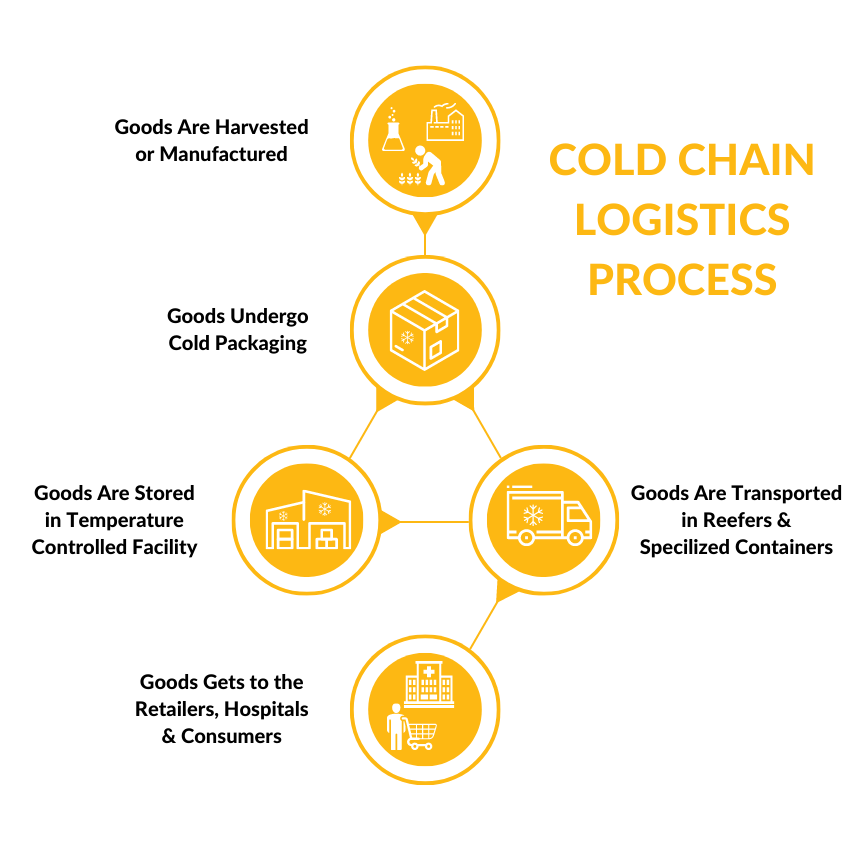
The journey begins with storage. Proper warehousing is the first and most critical link. It is not just about keeping things cold. Different products require specific, often narrow, temperature ranges. For instance, frozen foods typically need -18°C or lower, while many pharmaceuticals demand a controlled room temperature of 2°C to 8°C. Modern cold storage facilities use sophisticated refrigeration systems with redundant backups to prevent system failure. They are equipped with continuous temperature monitoring devices that log data and send alerts if parameters are breached. According to a report by the Global Cold Chain Alliance, the global capacity of refrigerated warehouses reached 719 million cubic meters in 2022, highlighting the scale of this infrastructure (来源: Global Cold Chain Alliance Capacity Report). The design of these warehouses also prioritizes efficient layout to minimize door-open time and maintain consistent temperatures throughout the facility.
## The Moving Link: Specialized Transportation and Packaging
Once products leave secure storage, they enter the most vulnerable phase: transportation. This segment involves a fleet of refrigerated trucks, containers, and air cargo units, collectively known as reefers. Each vehicle is essentially a mobile cold room. However, the equipment is only part of the solution. The packaging used inside is equally vital. Insulated shippers, phase change materials (PCMs), and vacuum insulated panels work together to create a stable microclimate for the product, protecting it against external temperature fluctuations during loading, unloading, or during a rare vehicle breakdown. The choice between active (mechanical) and passive (non-mechanical) cooling systems depends on the shipment’s duration, value, and sensitivity.
## The Digital Nervous System: Monitoring and Data Logging
You cannot manage what you do not measure. This old adage is the core principle of modern cold chain and logistics. Real-time monitoring has evolved from simple thermometer readings to Internet of Things (IoT) sensor networks. These small devices track not just temperature, but also humidity, light exposure, tilt, and shock. They transmit this data via cellular or satellite networks to a cloud platform, providing stakeholders with live visibility into their shipment’s condition. This data is not just for alerts. It creates an immutable chain of custody documentation, which is essential for regulatory compliance and quality assurance. If a temperature excursion occurs, the data helps determine the root cause and the potential impact on the product’s integrity.
## The Rulebook: Regulatory Compliance and Quality Standards
Operating in the cold chain space means navigating a complex web of regulations. These rules exist to protect public health. For food, standards like the FDA’s Food Safety Modernization Act (FSMA) in the US or the EU’s General Food Law set strict guidelines. For pharmaceuticals, Good Distribution Practice (GDP) is the global benchmark. These regulations mandate documented processes, qualified equipment, trained personnel, and a robust quality management system. Non-compliance is not an option. It can result in product recalls, massive fines, and irreparable damage to a brand’s reputation. Therefore, compliance is not a side activity. It is integrated into every operational step.
## The Human Factor: Training and Process Management
Even the most advanced technology can fail if people are not properly trained. The human element is the final, and often most variable, component. Staff at every touchpoint—from warehouse loaders to truck drivers to delivery personnel—must understand the critical nature of their role. They need training on proper handling procedures, such as minimizing door-open times, correct loading patterns to ensure air circulation, and emergency protocols for equipment failure. Process management ensures that these trained actions are consistent and repeatable. Standard Operating Procedures (SOPs) document every critical task, creating a culture of accountability and precision.
Based on my experience consulting for biotech firms, the single most common point of failure we see is not broken refrigerators, but a breakdown in communication and process during hand-off points between different logistics partners. A perfectly controlled warehouse shipment can be compromised in ten minutes on a loading dock without proper procedures.
### Comparing Active vs. Passive Temperature Control Solutions
Choosing the right temperature control method is a fundamental decision. Here is a clear comparison to guide that choice.
| Feature | Active Temperature Control (Reefers) | Passive Temperature Control (Insulated Containers) |
|---|---|---|
| Power Source | Requires continuous external power (fuel, electricity). | No external power needed. Relies on pre-conditioned PCMs. |
| Best For | Long-haul transport, high-value cargo, precise long-term control. | Short to medium hauls (up to 96 hrs), last-mile delivery, air freight. |
| Cost Structure | Higher operational cost (fuel, maintenance). | Higher upfront packaging cost, lower operational cost. |
| Flexibility & Sustainability | Limited route flexibility without power. Higher carbon footprint. | Highly flexible for multi-modal transport. Often uses recyclable materials. |
| Risk Factor | Mechanical failure, power loss. | Incorrect preconditioning, exceeding thermal buffer duration. |
## A 5-Step Guide to Implementing a Robust Cold Chain Monitoring System
Implementing visibility technology can seem daunting. Follow this practical step-by-step guide to get started.
STEP 1: DEFINE YOUR CRITICAL PARAMETERS. Identify what you need to monitor beyond just temperature. Is humidity critical for your product? Does it need to be protected from light? Does vibration during transit cause damage? List all the environmental factors that impact your product’s quality.
STEP 2: AUDIT YOUR CURRENT CHAIN. Map your entire supply chain from end to end. Identify all the touchpoints, hand-offs, and potential risk zones where temperature excursions are most likely to occur, such as airport tarmacs or transfer docks.
STEP 3: SELECT THE RIGHT SENSOR TECHNOLOGY. Choose sensors based on your needs from Step 1. Consider data connectivity. Do you need real-time (cellular/satellite) or is downloadable data loggers sufficient for your shipment durations and compliance needs?
STEP 4: INTEGRATE DATA INTO A CENTRAL PLATFORM. The data is useless if it sits in silos. Invest in a cloud-based platform that aggregates data from all shipments, provides clear dashboards, and automated alerting to key personnel via SMS or email.
STEP 5: ESTABLISH RESPONSE PROTOCOLS AND REVIEW. Technology provides the alert, but people must act. Create clear Standard Operating Procedures for what to do when an alarm is triggered. Regularly review excursion reports to identify systemic issues and improve processes continuously.
WARNING: COMMON COLD CHAIN MISCONCEPTION
A major mistake companies make is assuming that once a product is in a refrigerated truck or container, it is fully protected. The reality is that the loading and unloading processes are the highest-risk phases. A pallet of sensitive product left on a non-climate-controlled dock for 30 minutes on a hot day can experience a temperature excursion that invalidates its entire journey. This is known as the “first and last mile” problem, and it requires specific procedural controls and potentially temporary staging areas.
The global cold chain market is projected to grow significantly, driven by pharmaceutical demand and online grocery sales. A study by MarketsandMarkets estimates the market will reach $340.3 billion by 2025, up from $233.8 billion in 2020 (来源: MarketsandMarkets Cold Chain Market Report). This growth underscores the increasing importance of getting these logistics right.
In conclusion, cold chain and logistics is a complex symphony of physical infrastructure, digital tools, strict regulations, and human expertise. Success depends on excellence in all five components we have discussed. It is a field where attention to detail is not just beneficial, it is mandatory for safety and commercial viability.
COLD CHAIN IMPLEMENTATION CHECKLIST
PRODUCT ASSESSMENT: Clearly define the required temperature range and tolerances for your specific product.
INFRASTRUCTURE AUDIT: Verify the capability and certification of all storage and transportation partners.
TECHNOLOGY DEPLOYMENT: Implement appropriate monitoring devices for real-time or logged tracking.
DOCUMENTATION SYSTEM: Establish a system for recording and storing temperature data for compliance.
PERSONNEL TRAINING: Train all handlers on the importance of the cold chain and specific handling SOPs.
EMERGENCY PROTOCOLS: Develop and communicate clear procedures for responding to temperature excursions.
PARTNER ALIGNMENT: Ensure all third-party logistics providers are contractually bound to your quality standards.
CONTINUOUS REVIEW: Schedule regular reviews of monitoring data to identify and correct process weaknesses.