# The Ultimate Guide to Cold Chain Pharmaceutical Transport: 7 Critical Steps for Unbroken Integrity
The journey of a life-saving vaccine or a critical biologic therapy is a race against time and temperature. Cold chain pharmaceutical transport is not merely a logistics function. It is a sophisticated, high-stakes discipline that ensures medicines maintain their safety, efficacy, and quality from the moment they leave the manufacturing facility until they reach the patient. A single deviation can render a multi-million dollar batch worthless or, worse, pose a serious health risk. This guide delves deep into the complexities, technologies, and best practices that define world-class temperature-controlled logistics for pharmaceuticals.
Understanding the core challenge is essential. These products are not just “cold.” They have specific, narrow stability ranges defined as cold chain (2-8°C), frozen (-10°C to -25°C), or deep frozen (down to -70°C or lower for some mRNA vaccines). The goal of pharmaceutical cold chain logistics is to maintain this unbroken, documented thermal integrity throughout the entire supply chain.
## Why Cold Chain Integrity is Non-Negotiable
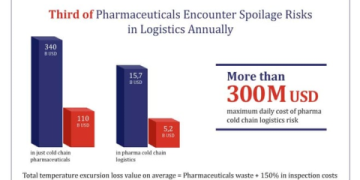
The consequences of a broken cold chain are severe and multi-faceted. Firstly, product degradation occurs. Temperature excursions can alter the chemical or physical structure of active pharmaceutical ingredients (APIs), making them less effective or completely inert. Secondly, patient safety is compromised. Degraded products can cause adverse reactions or fail to treat the condition, leading to poor health outcomes.

Financially, the losses are staggering. The average cost of a single temperature excursion event in the biopharma industry is estimated to be over $500,000 when considering product loss, investigation, and potential regulatory impacts (来源: IQVIA Institute Report). Furthermore, regulatory bodies like the FDA and EMA have stringent guidelines. Failure to comply can result in product recalls, warning letters, and devastating reputational damage that takes years to rebuild.
## Core Components of a Modern Cold Chain System
A robust system is built on multiple interdependent pillars. It starts with qualified packaging. Passive solutions use insulated containers with phase change materials (PCMs) like gel packs or dry ice. Active systems, used for longer hauls, incorporate battery-powered refrigeration units. The choice depends on transit duration, external ambient conditions, and product sensitivity.
Real-time monitoring is the digital nerve center. Modern data loggers and IoT sensors do more than record temperature. They track location, humidity, light exposure, and shock, transmitting this data to a cloud platform for real-time visibility. This shift from retrospective “black box” analysis to proactive intervention is a game-changer.
Finally, carrier and partner qualification is critical. Not all logistics providers are equal. Partners must have proven protocols, trained personnel, and contingency plans specifically for healthcare products, adhering to standards like GDP (Good Distribution Practice).
## Critical Technologies Shaping the Future
The landscape is being transformed by technology. Internet of Things (IoT) sensors provide granular, real-time data streams, enabling predictive alerts before an excursion occurs. Blockchain technology is emerging as a powerful tool for creating immutable, transparent audit trails, enhancing trust across complex supply chains.
Artificial intelligence and machine learning are moving to the forefront. These systems analyze vast datasets from historical shipments, weather patterns, and traffic to optimize routes, predict potential failure points, and improve packaging design. According to a recent study by DHL, AI-driven predictive analytics can reduce cold chain logistics risks by up to 30% (来源: DHL Logistics Trend Radar).
## Choosing Your Cold Chain Packaging: A Strategic Comparison
Selecting the right packaging is a fundamental decision. The choice between passive and active systems hinges on several factors. Here is a clear comparison to guide your strategy.
| Feature | Passive Packaging (e.g., Insulated Shipper with PCMs) | Active Packaging (e.g., Powered Refrigerated Container) |
|---|---|---|
| Power Source | Phase Change Materials (PCMs) pre-conditioned at specific temperatures. | Battery or mains electricity, often with backup power. |
| Duration of Control | Typically 24 to 96 hours, dependent on design and ambient conditions. | Several days to weeks, capable of maintaining precise temperature indefinitely with power. |
| Cost Profile | Lower upfront capital cost, but recurring per-shipment material and conditioning costs. | High upfront capital cost, but lower variable cost per trip for high-volume lanes. |
| Best Use Case | Short to medium-haul transport (e.g., airport-to-hospital), clinical trial sample distribution. | Long-distance international sea/air freight, transport of ultra-high-value or critical stock. |
| Flexibility | High. Boxes can be stored flat and assembled as needed for different payloads. | Low. Units are fixed assets dedicated to specific temperature ranges and routes. |
## The 7-Step Operational Guide to Flawless Execution
Based on my experience auditing dozens of supply chains, success lies in a meticulous, step-by-step process. Here is a practical operational guide.
STEP 1: CONDUCT A RISK ASSESSMENT. Map the entire journey from origin to destination. Identify all hand-off points, potential delays, and extreme ambient conditions. Document every risk.
STEP 2: SELECT AND QUALIFY PACKAGING. Based on the risk assessment, choose a packaging solution. Then, formally qualify it through standardized testing (like ISTA 7D) under simulated worst-case conditions.
STEP 3: IMPLEMENT REAL-TIME MONITORING. Deploy calibrated, GPS-enabled data loggers with cellular connectivity. Set clear, product-specific alert thresholds for temperature deviations.
STEP 4: TRAIN ALL PERSONNEL. Everyone who touches the shipment—packers, warehouse staff, drivers—must understand the product’s sensitivity and their role in maintaining the cold chain. This is often the weakest link.
STEP 5: ESTABLISH CLEAR PROTOCOLS FOR EXCURSIONS. Define exactly what happens when an alert is triggered. Who is notified? What are the immediate containment actions? What is the product quarantine and investigation process?
STEP 6: DOCUMENT EVERYTHING. Maintain a complete, audit-ready record of temperature data, chain of custody documents, calibration certificates, and deviation reports.
STEP 7: CONDUCT REGULAR REVIEWS AND AUDITS. Periodically review performance data, audit your carriers and warehouses, and update your risk assessments and protocols based on new findings or technologies.
## Common Pitfalls and How to Avoid Them
Even with the best technology, human and process errors can cause failures. Here is a crucial warning on the most common mistakes.
WARNING: THE MOST COMMON COLD CHAIN FAILURES
A major pitfall is assuming the packaging system is foolproof. Teams often neglect to pre-condition phase change materials (PCMs) to the exact required temperature, leading to rapid failure. Another critical error is poor communication during hand-offs. A shipment left on a loading dock for an extended period because the receiving team was not alerted is a classic failure point. Finally, relying on outdated “ship and pray” methods with no real-time visibility guarantees you will discover problems too late. Proactive management is the only acceptable approach.
## Your Actionable Cold Chain Integrity Checklist
Before your next critical shipment, use this checklist to ensure all bases are covered. Do not proceed unless you can confirm each point.
CONFIRM the product’s exact temperature stability range and any special handling requirements.
VALIDATE that the selected packaging system has been formally qualified for the specific transit duration and expected ambient profile.
VERIFY that all temperature monitoring devices are calibrated, activated, and transmitting data before dispatch.
COMMUNICATE the shipment’s critical status and tracking details to all parties in the supply chain, including the final recipient.
DOCUMENT the packing process, including the conditioning state of all PCMs and the serial numbers of monitoring devices.
ESTABLISH and TEST the escalation protocol for temperature excursion alerts before the shipment begins its journey.
CONDUCT a post-shipment review of all data and documentation to identify any areas for process improvement.
In conclusion, mastering cold chain pharmaceutical transport requires a blend of robust technology, rigorous processes, and a culture of accountability. It is a dynamic field where continuous improvement is not just beneficial but mandatory. By viewing the cold chain as an integrated, monitored system rather than a series of isolated steps, pharmaceutical companies and logistics providers can protect patient health, safeguard valuable products, and build a reputation for unwavering reliability. The journey is complex, but with the right approach, the integrity of every vital shipment can be assured.