# The Ultimate Guide to the Biopharma Supply Chain: 7 Expert Strategies for Resilience
The biopharma supply chain is the critical, beating heart of the life sciences industry. It is not merely a logistics network. It is a complex, high-stakes system responsible for moving highly sensitive, temperature-controlled, and often life-saving therapies from development labs to patients worldwide. A single failure in this chain can mean the difference between a successful treatment and a devastating loss. This guide provides an authoritative, in-depth look at the modern biopharma supply chain, its unique challenges, and the expert strategies leading companies use to build resilience and ensure patient safety.
UNDERSTANDING THE UNIQUE COMPLEXITY OF BIOPHARMA LOGISTICS
What makes the biopharma supply chain so distinct from other industries? The answer lies in the products themselves. Unlike small-molecule pills, biologics—such as monoclonal antibodies, vaccines, and cell therapies—are large, complex molecules derived from living organisms. They are incredibly sensitive to environmental conditions. Most require strict, unbroken temperature control, often at ultra-low ranges like -80°C. This cold chain management is non-negotiable. A temperature excursion can render a multi-thousand-dollar vial completely ineffective, posing a direct risk to patient health and causing massive financial waste.
Furthermore, the regulatory landscape is exceptionally stringent. Every step, from raw material sourcing to final delivery, must comply with Good Distribution Practices (GDP) and other global regulations set by bodies like the FDA and EMA. Traceability is paramount. Companies must be able to track the precise location, temperature history, and chain of custody for every single product unit. This level of oversight is essential for patient safety, quality assurance, and rapid response in the event of a recall.

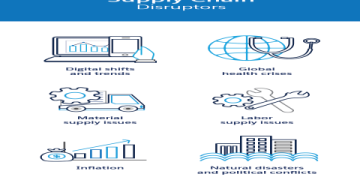
THE TOP 5 CHALLENGES FACING MODERN BIOPHARMA SUPPLY CHAINS
Navigating this landscape is fraught with obstacles. Here are the five most pressing challenges:
1. COLD CHAIN COMPLEXITY: Maintaining end-to-end temperature integrity across global transit, through multiple handoffs and transport modes, is a monumental technical and operational task.
2. REGULATORY FRAGMENTATION: Differing regulations across countries and regions create a maze of compliance requirements, slowing down market access and increasing administrative costs.
3. DEMAND VOLATILITY AND FORECASTING: Predicting demand for novel therapies is difficult. Launch volumes can be unpredictable, and demand spikes (as seen during pandemics) can strain capacity to its limits.
4. SUPPLIER RELIANCE AND VISIBILITY: The industry relies on a network of contract manufacturers, logistics providers, and raw material suppliers. A disruption at any single node can halt the entire flow.
5. PRODUCT SECURITY AND COUNTERFEITING: High-value products are targets for theft and counterfeit operations, necessitating robust security and serialization protocols.
A critical data point underscores the financial impact: a 2023 survey by the IQVIA Institute for Human Data Science found that supply chain inefficiencies and product losses cost the biopharmaceutical industry an estimated $35 billion annually. (来源: IQVIA Institute)
BUILDING RESILIENCE: A 7-STEP STRATEGIC FRAMEWORK
Overcoming these challenges requires a proactive, strategic approach. Here is a seven-step framework to fortify your biopharma supply chain.
STEP 1: IMPLEMENT END-TO-END DIGITAL VISIBILITY
Move beyond periodic updates. Implement IoT sensors and a centralized control tower platform that provides real-time data on location, temperature, humidity, and shock for every shipment. This enables proactive intervention.
STEP 2: DIVERSIFY YOUR SUPPLY NETWORK
Over-reliance on single-source suppliers or specific geographic regions is a key vulnerability. Actively qualify alternative suppliers and contract manufacturers to build redundancy.
STEP 3: ADOPT ADVANCED PREDICTIVE ANALYTICS
Leverage AI and machine learning tools to analyze historical data, market signals, and clinical trial progress. This improves demand forecasting accuracy for new therapy launches.
STEP 4: STANDARDIZE AND OPTIMIZE COLD CHAIN PACKAGING
Work with packaging engineers to select the right insulated shipper for each lane and product profile. Conduct rigorous pre-qualification testing under simulated worst-case conditions.
STEP 5: FOSTER STRATEGIC PARTNER COLLABORATION
Treat key logistics providers and CMOs as strategic partners, not just vendors. Share data, conduct joint risk assessments, and co-develop contingency plans.
STEP 6: INVEST IN SERIALIZATION AND TRACK-AND-TRACE
Go beyond compliance. Use unique product identifiers (like 2D barcodes) to build a fully digital thread, enhancing security, enabling faster recalls, and combating counterfeits.
STEP 7: CONDUCT REGULAR STRESS TESTS AND SCENARIO PLANNING
Do not wait for a real crisis. Regularly run simulation exercises for scenarios like port closures, supplier bankruptcy, or extreme weather events to identify gaps in your response plans.
Based on my experience consulting for mid-sized biotechs, the transition from a reactive to a proactive supply chain mindset is the single biggest hurdle. One client avoided a major loss by acting on a real-time temperature alert from their new visibility platform, allowing them to reroute a critical shipment before it was compromised. That single event justified their entire technology investment.
COMPARING KEY SUPPLY CHAIN TECHNOLOGY SOLUTIONS
Choosing the right technology is crucial. The table below contrasts two primary categories of solutions.
| FEATURE | STAND-ALONE MONITORING DEVICES | INTEGRATED CONTROL TOWER PLATFORM |
|---|---|---|
| Core Function | Records and stores temperature/location data for a single shipment. Data is downloaded after delivery. | Provides real-time, centralized visibility and analytics for the entire supply network via a cloud dashboard. |
| Data Access | Retrospective; after-the-fact. Alerts come too late for intervention. | Real-time; proactive. Enables live tracking and immediate corrective action. |
| Integration | Low. Data silos exist. Manual data aggregation is required. | High. Can integrate with ERP, WMS, and partner systems for a single source of truth. |
| Best For | Proving compliance for a specific shipment or lane where real-time action is less critical. | Managing complex, global networks, mitigating risks proactively, and driving strategic optimization. |
A COMMON MISTAKE TO AVOID
WARNING: A prevalent mistake is treating supply chain management as a purely operational or cost-center function. In biopharma, the supply chain is a core strategic asset directly linked to clinical trial success, commercial launch velocity, and patient outcomes. Under-investing in talent and technology here to save on short-term costs inevitably leads to far greater losses through clinical delays, product waste, and compliance failures. The supply chain team must have a seat at the strategic table from early development stages.
THE FUTURE: TRENDS SHAPING THE NEXT GENERATION BIOPHARMA SUPPLY CHAIN
The future is being shaped by powerful trends. Personalized medicine, like CAR-T cell therapies, demands a “vein-to-vein” supply chain model that treats a patient’s own cells as the starting material. This requires unprecedented coordination and speed. Artificial intelligence is moving from forecasting to autonomous decision-making, such as dynamically rerouting shipments based on weather or traffic. Furthermore, sustainability is becoming a regulatory and investor priority, pushing the industry toward greener packaging, optimized transport to reduce carbon footprint, and circular economy principles. Another significant trend is the move towards regionalization—building manufacturing and supply networks closer to key patient populations to reduce geopolitical risk and improve agility, a shift accelerated by recent global disruptions. (来源: McKinsey & Company analysis on biopharma operations)
FINAL CHECKLIST FOR A RESILIENT BIOPHARMA SUPPLY CHAIN
Use this actionable checklist to assess and strengthen your operations:
– Confirm real-time visibility is active for all critical shipments, not just a select few.
– Validate that your cold chain packaging is qualified for the actual worst-case transit conditions it will face.
– Document and regularly update a detailed map of your tier-1 and tier-2 supplier network, including single points of failure.
– Ensure your serialization and traceability systems meet the requirements of all markets you serve or plan to enter.
– Schedule and conduct a full supply chain disruption simulation exercise at least once per calendar year.
– Review and align your key performance indicators (KPIs) to focus on patient-centric metrics like delivery reliability and quality, not just cost.
– Establish a formal governance process for sharing supply chain risk and performance data with executive leadership and R&D teams.
Mastering the biopharma supply chain is a continuous journey of adaptation and investment. By embracing digital transformation, fostering deep collaboration, and prioritizing resilience over mere efficiency, organizations can transform this complex network from a source of risk into a definitive competitive advantage that reliably delivers hope and health to patients.